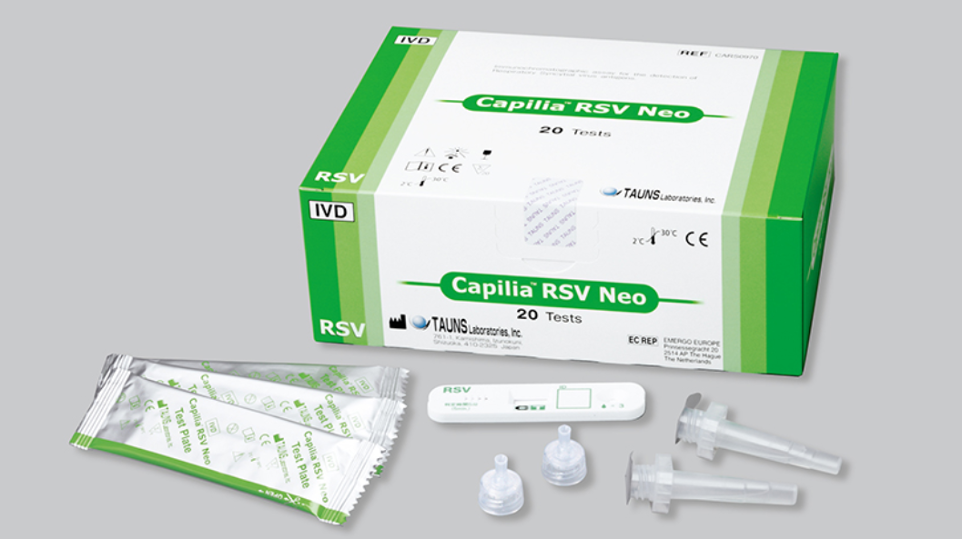
Capilia™ RSV Neo
Capilia™ RSV Neo is a rapid and accurate immunoassay test that detects RSV antigens directly from nasal swab or nasal aspirate samples. The test is easy to use and can be performed in just 3-5 minutes.
Capilia™ RSV Neo represents a significant leap forward in RSV detection, offering a combination of speed, accuracy, ease of use, versatility, and cost-effectiveness that surpasses traditional methods. Its transformative potential lies in its ability to empower healthcare professionals to effectively diagnose RSV infections, promote timely treatment, and contribute to better patient outcomes. As a cornerstone of RSV diagnostic strategies, Capilia™ RSV Neo paves the way for a future where RSV is effectively managed and controlled, minimizing its impact on global health.
Rapid and Accurate Detection of Respiratory Syncytial Virus (RSV) Antigen
Product Overview
This rapid test kit provides a convenient and reliable method for detecting the presence of RSV antigens in nasopharyngeal aspirate (NPA) or nasopharyngeal swabs (NPS) samples within 3-5 minutes. The test utilizes advanced platinum-gold colloid nanotechnology to deliver sensitive and accurate results, making it an ideal tool for diagnosing RSV infections in both pediatric and adult patients.
Key Features
- Rapid Results: Obtain results in as little as 3-5 minutes, enabling prompt treatment decisions and infection control measures.
- High Sensitivity and Specificity: Accurately detect RSV antigens with a high degree of sensitivity and specificity, ensuring confidence in test results.
- Easy to Use: Simple and straightforward procedure requires minimal training and time to perform.
- Easy to Read: Clear and distinct black lines indicate positive or negative results, eliminating ambiguity in interpretation.
- Versatile Application: Can be used to test both NPA and NPS samples, catering to a wider range of clinical settings.
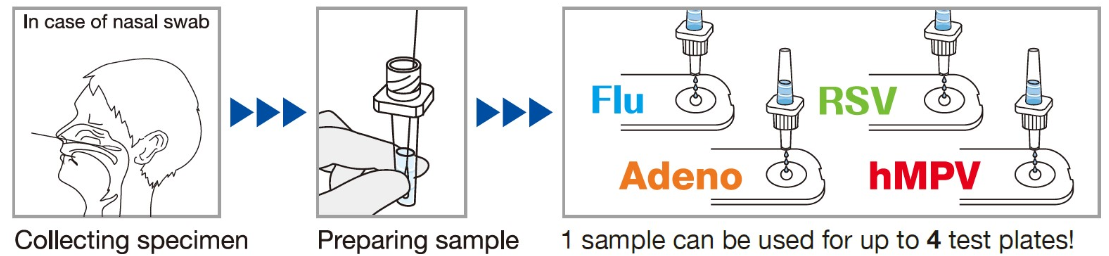
- Multiplex Testing Capability: A single sample can be used to simultaneously test for RSV, influenza virus A/B, adenovirus, and human metapneumovirus (hMPV), providing comprehensive respiratory viral screening.
- Shared Extraction Buffer: Capilia series extraction buffer can be used for all four tests, streamlining the workflow and reducing costs.
Benefits
- Improved Patient Care: Timely diagnosis of RSV infections facilitates appropriate treatment and management, leading to better patient outcomes.
- Infection Control: Early identification of RSV cases enables effective infection control measures to prevent the spread of the virus.
- Reduced Healthcare Costs: Prompt diagnosis and treatment can minimize the severity of RSV infections, potentially reducing hospitalizations and healthcare costs.
Applications
- Healthcare Settings: Clinicians in hospitals, clinics, and urgent care centers can utilize the rapid RSV antigen test to make informed treatment decisions and implement infection control strategies.
- Home Testing: Healthcare providers may recommend home-based RSV testing for high-risk individuals, such as infants and young children, to facilitate early diagnosis and intervention.
- Surveillance and Research: Public health authorities and researchers can employ the rapid RSV antigen test for surveillance purposes to monitor RSV activity and gather valuable data for epidemiological studies.
Conclusion
This rapid test kit offers a valuable solution for the rapid and accurate detection of RSV infections, contributing to improved patient care, effective infection control, and optimized healthcare resource utilization. Its ease of use, versatility, and multiplex testing capability make it a versatile tool for a wide range of applications in various clinical settings.
SPECIMEN COLLECTION AND PREPARATION
Methods of specimen collection
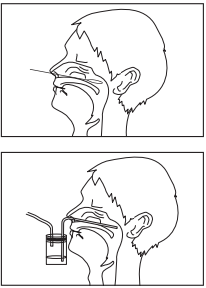
Nasal swab sampling
- Firmly insert a nasal swab into the nasal cavity.
- Rotate the swab several times while applying gentle pressure to collect mucosal epithelium from the nasal turbinates.
Nasal aspirate sampling
- Connect one tube of a suction trap to a suction pump and the other tube to a nasal cavity through an external nostril.
- Turn on the suction pump to collect nasal discharge aspirate in the suction trap.
- Soak a swab in the collected nasal aspirate, ensuring it absorbs the liquid well.
- If using a micropipette or other instrument to collect nasal aspirate, dilute the aspirate twofold with physiological saline.
- Sample 200 μL of the diluted nasal aspirate for testing.
- Sample preparation
- Precautions for sample preparation
For a highly viscous sample that can cause filter clogging, dilute the sample twofold with physiological saline before use.
TEST PROCEDURE
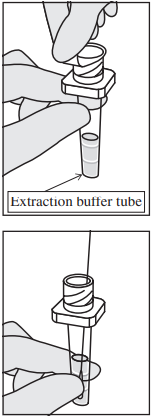
- Securely attach the provided nozzle (with a filter) to the top of the extraction buffer tube.
- Hold the middle of the extraction buffer tube firmly and dispense 3 drops (approximately 80-120 μL) of the sample onto the designated sample placement area of the test plate. Maintain a vertical orientation of the tube and avoid contact between the nozzle tip and the sample placement area.
- Allow the test plate to sit for 3-5 minutes, then observe the reading area. Interpret the results according to the "READING TEST RESULTS" guidelines.
Sample Placement Precautions
To ensure accurate results, it is crucial to dispense the correct amount of sample onto the test plate. Exceeding the recommended volume can prolong the reaction time and potentially lead to false negative results. The excess sample dilutes the colloidal platinum-gold labeled antibody, affecting the sensitivity of the test and potentially preventing the appearance of visible lines at the [C] and [T] positions within the specified judgment time. In some cases, faint lines may be visible, further complicating the interpretation of the results. Therefore, carefully follow the instructions and dispense only 3 drops (approximately 80-120 μL) of the sample onto the designated sample placement area. This will ensure optimal test performance and accurate results.
Limitations
- Diagnostic Confirmation: While this rapid test provides a convenient and timely indication of RS virus infection, it is considered a screening tool and not a definitive diagnostic method. A definitive diagnosis should always be made by a healthcare provider based on a comprehensive evaluation, including clinical symptoms, patient history, and potentially, confirmatory laboratory tests such as RT-PCR.
- Moisture Sensitivity: The test plate's integrity and performance are susceptible to moisture. Once the packaging is opened, the test plate should be used immediately to prevent exposure to ambient moisture, which could degrade the quality of the test components and compromise the accuracy of the results.
- Intended Use: This rapid test is exclusively designed for in vitro diagnostic purposes and should not be used for any other applications. Improper or unintended use of the test may yield unreliable results and could lead to misinterpretations.
- Procedural Adherence: To ensure the validity of the test results, it is imperative to strictly adhere to the operational instructions provided in the package insert. Deviating from the prescribed procedure may invalidate the test and render the results inconclusive or inaccurate.
- Safety Precautions: The extraction buffer contains sodium azide, a potentially hazardous substance. In the event of accidental contact with eyes, mouth, or skin, immediately flush the affected area thoroughly with water and seek medical attention if necessary.