Vaccinia Capping Enzyme
| Catalog | Product Name | Size | KU/mL |
|---|---|---|---|
| HBP000606 | Vaccinia virus Capping Enzyme | 0.5KU/0.05mL | 10 |
| HBP000607 | Vaccinia virus Capping Enzyme | 2KU/0.2mL | 10 |
| HBP000608 | Vaccinia virus Capping Enzyme | 100KU/10mL | 10 |
| HBP000609 | Vaccinia virus Capping Enzyme | 1000KU/100mL | 10 |
| HBP000610 | Vaccinia virus Capping Enzyme | 10000KU/1000mL | 10 |
The Vaccinia Capping Enzymeemerges as a pivotal player in the intricate landscape of molecular biology, contributing significantly to the fundamental processes of RNA metabolism. At the heart of eukaryotic mRNA processing, this enzyme takes center stage in the capping of mRNA molecules, a crucial step for their stability, transport, and translation. By catalyzing the addition of a 7-methylguanosine cap to the 5' end of nascent RNA, the Vaccinia Capping Enzyme not only safeguards mRNA from premature degradation but also orchestrates the intricate dance of cellular events that dictate gene expression. Its role extends beyond mere capping; it becomes a regulatory conductor, influencing the fate and functionality of mRNA in the cellular orchestra. Researchers harness the power of the Vaccinia Capping Enzyme in unraveling the mysteries of gene expression, providing a key to understanding cellular processes and paving the way for innovations in biotechnology and therapeutic interventions. In the complex symphony of molecular biology, the Vaccinia Capping Enzyme stands as a conductor, directing the harmonious expression of genetic information and unlocking the secrets encoded in the language of RNA.
Vaccinia Capping Enzyme
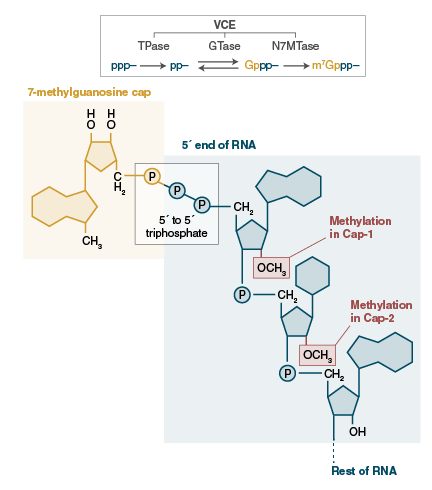
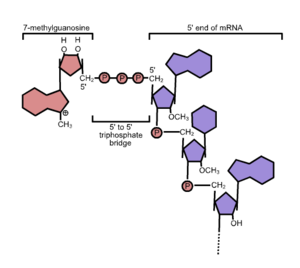
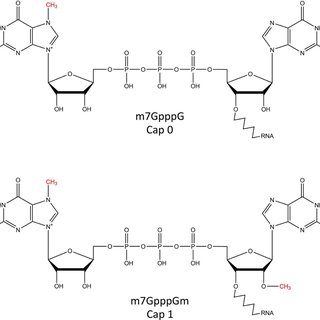
The Vaccinia virus capping enzyme is obtained from a recombinant E. coli strain containing the genes responsible for the Vaccinia capping enzyme. Comprising two subunits (D1 and D12), this singular enzyme exhibits three distinct enzymatic activities: RNA triphosphatase and guanylyltransferase via the D1 subunit, and guanine methyltransferase through the D12 subunit. The Vaccinia virus Capping Enzyme is proficient in facilitating the formation of the cap structure, enabling the specific attachment of the 7-methylguanylate cap structure (m7Gppp, Cap 0) to the 5' end of RNA. This cap structure (Cap 0) plays a pivotal role in enhancing mRNA stabilization, transport, and translation in eukaryotes. Employing enzymatic reactions to cap RNA represents an effective and straightforward method, significantly enhancing RNA stability and translation for applications such as in vitro transcription, transfection, and microinjection.
| Component | Specification |
|---|---|
| Product | Vaccinia virus Capping Enzyme |
| Storage | -20℃ for storage (Avoid repeated freeze-thaw cycles) |
| Storage buffer | 20 mM Tris-HCl (pH 8.0), 100 mM NaCl, 1 mM DTT, 0.1mM EDTA, 0.1% Triton X-100, 50% glycerol. |
| Unit Definition | One unit of Vaccinia virus Capping Enzyme is defined as the amount of enzyme required to incorporate 10 pmol of GTP into an 80 nt transcript in 1 hour at 37°C. |
Quality Control
- Exonuclease: 10 U of Vaccinia virus Capping Enzyme with 1 μg λ-Hind III digest DNA at 37 ℃ for 16 hours yields no degradation as determined by agarose gel electrophoresis.
- Endonuclease: 10 U of Vaccinia virus Capping Enzyme with 1 μg λDNA at 37 ℃ for 16 hours yields no degradation as determined by agarose gel electrophoresis.
- Nickase: 10 U of Vaccinia virus Capping Enzyme with 1 μg pBR322 at 37 ℃ for 16 hours yields no degradation as determined by agarose gel electrophoresis.
- RNase: 10 U of Vaccinia virus Capping Enzyme with 1.6 μg MS2 RNA for 4 hours at 37 ℃ yields no degradation as determined by agarose gel electrophoresis.
- E.coli DNA: 10 U of Vaccinia virus Capping Enzyme is screened for the presence of E. coli genomic DNA using TaqMan qPCR with primers specific for the E. coli 16S rRNA locus. The E. coli genomic DNA contamination is≤0.1 pg/10 U.
- Bacterial Endotoxin: LAL-test, according to Chinese Pharmacopoeia IV 2020 edition, gel limit test method, general rule (1143). Bacterial endotoxin content should be ≤10 EU/mg.
Reaction system and conditions
Capping Protocol (reaction volume: 20 μL)
This procedure is applicable to the capping reaction of 10μg RNA (≥100 nt) and it can be scaled up according to experimental demands.
- Combine 10 μg RNA and Nuclease-free H2O in a 1.5 ml microfuge tube to a final volume of 15.0 µL.
- Heat at 65℃ for 5 minutes followed by ice bath for 5 minutes.
- Add the following components in the order specified:
| Component | Volume |
|---|---|
| Denatured RNA (≤10 μg, length≥100 nt) | 15 μL |
| 10×Capping Buffer* | 2 μL |
| GTP (10 mM) | 1 μL |
| SAM (2 mM) | 1 μL |
| Vaccinia virus Capping Enzyme (10 U/μL) | 1 μL |
| KCl, 10 mM MgCl2, 10 mM DTT, (25℃, pH 8.0) | - |
- 10×Capping Buffer:
| Component | Final concentration |
|---|---|
| Tris-HCl (pH 8.0) | 200 mM |
| NaCl | 1000 mM |
| DTT | 10 mM |
| EDTA | 1 mM |
| Triton X-100 | 1% |
| Glycerol | 50% |
4. Incubate at 37°C for 30 minutes, RNA is now capped and ready for downstream applications.
This protocol is designed to label RNA containing a 5´ triphosphate and it can be scaled up according to demands. The efficiency of label incorporation will be impacted by the molar ratio of RNA: GTP, as well as the GTP content in RNA samples
- Combine appropriate amount of RNA and Nuclease-free H2O in a 1.5 ml microfuge tube to a final volume of 14.0 µL.
- Heat at 65℃ for 5 minutes followed by ice bath for 5 minutes.
- Add the following components in the order specified
Applications:
- Capping mRNA to improve translation efficiency
- Labeling the 5' end of mRNA
This reformulation is more concise and easier to read, and it also more accurately reflects the two main applications of the Vaccinia virus Capping Enzyme.
The original formulation, "Capping mRNA prior to translation assays/in vitro translation," is a bit cumbersome and repetitive. The word "translation" is used twice, and the phrase "in vitro translation" is redundant.
The reformulated version, "Capping mRNA to improve translation efficiency" is more concise and to the point. It also more accurately reflects the fact that the Vaccinia virus Capping Enzyme can be used to cap mRNA for a variety of purposes, including translation assays, in vitro translation, and other downstream applications.
The second application, "Labeling 5' end of mRNA," is also more concise and easier to read than the original formulation, "Labeling 5´ end of mRNA."