OptiPrep™ Application Sheet S47a
Intracellular exocytic vesicle trafficking and exocyst complex – ashort methodological summary
| Page 1 |
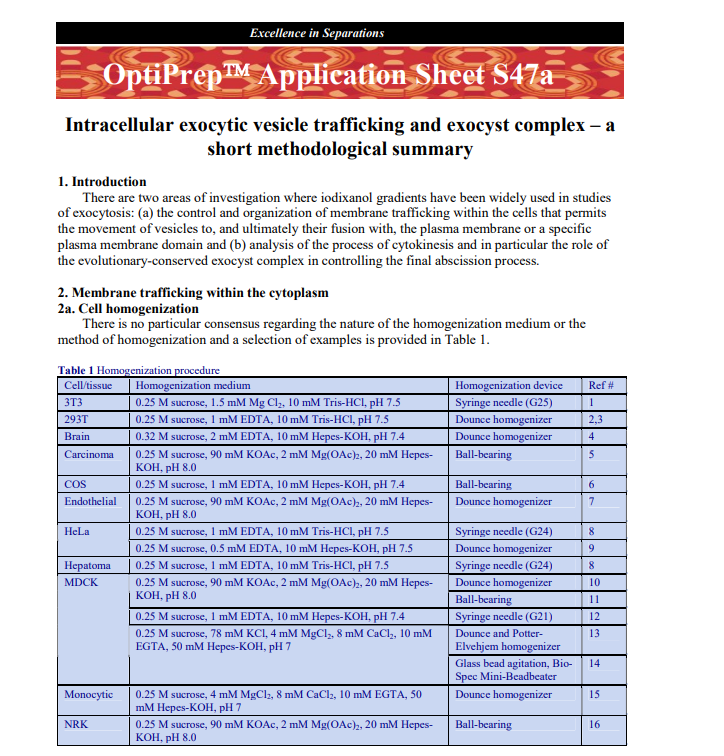
1 Intracellular exocytic vesicle trafficking and exocyst complex – ashort methodological summary 1. IntroductionThere are two areas of investigation where iodixanol gradients have been widely used in studiesof exocytosis: (a) the control and organization of membrane trafficking within the cells that permitsthe movement of vesicles to, and ultimately their fusion with, the plasma membrane or a specificplasma membrane domain and (b) analysis of the process of cytokinesis and in particular the role ofthe evolutionary-conserved exocyst complex in controlling the final abscission process.2. Membrane trafficking within the cytoplasm2a. Cell homogenizationThere is no particular consensus regarding the nature of the homogenization medium or themethod of homogenization and a selection of examples is provided in Table 1. Table 1 Homogenization procedureCell/tissueHomogenization mediumHomogenization deviceRef #3T30.25 M sucrose, 1.5 mM Mg Cl2, 10 mM Tris-HCl, pH 7.5Syringe needle (G25)1293T0.25 M sucrose, 1 mM EDTA, 10 mM Tris-HCl, pH 7.5Dounce homogenizer2,3Brain0.32 M sucrose, 2 mM EDTA, 10 mM Hepes-KOH, pH 7.4Dounce homogenizer4Carcinoma0.25 M sucrose, 90 mM KOAc, 2 mM Mg(OAc)2, 20 mM Hepes-KOH, pH 8.0Ball-bearing5COS0.25 M sucrose, 1 mM EDTA, 10 mM Hepes-KOH, pH 7.4Ball-bearing6Endothelial0.25 M sucrose, 90 mM KOAc, 2 mM Mg(OAc)2, 20 mM Hepes-KOH, pH 8.0Dounce homogenizer70.25 M sucrose, 1 mM EDTA, 10 mM Tris-HCl, pH 7.5Syringe needle (G24)8HeLa0.25 M sucrose, 0.5 mM EDTA, 10 mM Hepes-KOH, pH 7.5Dounce homogenizer9Hepatoma0.25 M sucrose, 1 mM EDTA, 10 mM Tris-HCl, pH 7.5Syringe needle (G24)8Dounce homogenizer100.25 M sucrose, 90 mM KOAc, 2 mM Mg(OAc)2, 20 mM Hepes-KOH, pH 8.0Ball-bearing110.25 M sucrose, 1 mM EDTA, 10 mM Hepes-KOH, pH 7.4Syringe needle (G21)12Dounce and Potter-Elvehjem homogenizer13MDCK0.25 M sucrose, 78 mM KCl, 4 mM MgCl2, 8 mM CaCl2, 10 mMEGTA, 50 mM Hepes-KOH, pH 7Glass bead agitation, Bio-Spec Mini-Beadbeater14Monocytic0.25 M sucrose, 4 mM MgCl2, 8 mM CaCl2, 10 mM EGTA, 50mM Hepes-KOH, pH 7Dounce homogenizer15NRK0.25 M sucrose, 90 mM KOAc, 2 mM Mg(OAc)2, 20 mM Hepes-KOH, pH 8.0Ball-bearing16 ♦ Any cocktail of protease inhibitors may be added to the homogenization medium 2b Pre-gradient processingIn most cases the fraction that is applied to the gradient, or incorporated into the gradient, is apost-nuclear supernatant (PNS). The single centrifugation may be carried out at any g-force from 500-1000 g for 5-10 min, although occasionally a post-heavy mitochondrial supernatant (3000 g) is used(see e.g. ref 6). Sometimes a more extensive differential centrifugation is carried out, for example anMDCK cell homogenate was centrifuged sequentially at 1000 g for 10 min, 5,000 g for 40 min andthen 100,000 g for 2 h; the pellet from the last centrifugation was applied to the gradient [14]. Rathermore rarely the differential centrifugation may be more extensive: e.g. 1000 g for 10 min, 5000 g for10 min, 10,000 g for 20 min, 15,000 g for 20 min and then the final pellet at 100,000 g for 1 h was Excellence in Separations
| Page 2 |
2 resuspended and incorporated into a continuous gradient [13]. The advantage of using a PNS is thereduction in time between homogenization and gradient and losses of vesicles will be minimal. Thedisadvantage is the presence of all the other large organelles (mitochondria, lysosomes, peroxisomes)that will band in the denser regions of the gradient. Maybe a single 5000 g centrifugation, which willeliminate most of the mitochondria and some of the peroxisomes and lysosomes, might be a usefulcompromise.2c. Self-generated gradientsThe 10%-20%-30% (w/v) iodixanol start-ing format for the preparation of gradients thatare close to linear was first introduced byYeaman et al [16] in 2001. These gradients arebest prepared in a vertical or near-vertical rotorwith a tube volume of no more than 13 ml; forexample the Beckman VTi65.1 or NVT65 orthe Sorvall 65V13. Smaller volume rotors (5-6 ml tubes) such as the Beckman VTi65.2 or NVT65.2or the Sorvall 70V6 are equally acceptable. The method has often been by Yeaman and his colleaguesin studies on the influence of the Sec6/8 exocyst complex in controlling membrane vesicle delivery tothe plasma membrane of polarized cells [17,18]. In more recent experiments the gradient wasmodified: an MDCK cell PNS was adjusted to 30% iodixanol and layers of 25%, 20%, 15% and 10%(w/v) iodixanol layered on top, otherwise the centrifugation conditions were the same [5]. Thegradient clearly resolved the Sec8 complex in the denser from the lighter Na+/K+-ATPase, while thepaxillin marker was biphasic with only the lighter peak co-migrating with the Sec8 (see Figure 1). Thestandard 10%-20%-30% (w/v) iodixanol starting format was used in a study of the relationshipbetween the Sec3 containing exocyst complex and desmosome assembly [11].Kolesnikova et al [8] used the 10-20-30% iodixanol gradient to monitor the translocation of theVP40 matrix protein of Marburg virus in infected cells. At 7 h post-infection most of the VP40 wasassociated with the small vesicle fraction but as the infection progressed (up to 24 h) the gradientpermitted the demonstration of a shift through the endosomal/ER zone to the plasma membrane.A HeLa cell PNS was fractionated on the 10%-20%-30% (w/v) iodixanol gradient, with the PNSonly in 30% layer and centrifuged at 330,000 g for 3 h: M-Sec and RalA formed a clear biphasicdistribution, only the denser material co-fractionating with the Sec6/8 exocyst complex [9]. Using thesame gradient and centrifugation format Chen et al [6] studied exocyst regulation of vesicle deliveryto the centrosome prior to cytokinesis in COS cells: they observed a co-banding of RalA with TfR andRab11 but not with early endosomes, Golgi or cytosol markers.An additional layer of 15% iodixanol was inserted by Wang et al [12] who found that in PALS1knockdown cells there was a significant shift in the banding of Sec8 and E-cadherin compared towild-type cells. ♦ Similar self-generated gradients have also been used for the analysis of secretory proteins and the exocytic process for Drosophila [19] and in the study of the secretion of bone matrix proteins byosteoclasts [20].2d. Gradients in swinging bucket rotorsLeblanc et al [1] working with 3T3 cells used a three-layer 10-30% (w/v) iodixanol gradient,again with the PNS in the densest layer in small volume (4 ml) swinging-bucket rotor at 260,000g for3 h. A continuous gradient will form during the centrifugation mainly by diffusion, although someself-generation may also occur. The separation of dense small vesicles from lighter endosomes andplasma membrane was similar to that in ref 8.Other gradients have conformed to the traditional format of top-loading of pre-formed gradientscentrifuged at lower g-forces. A microsomal fraction placed on top of a continuous 10-40% (w/v) High densityLow density PaxillinSec 8Na/K-ATPase Figure 1: Approximate positions of protein markers from aMDCK cell PNS in the iodixanol gradient (adapted from ref 5).For more information see text.
| Page 3 |
3 iodixanol gradient, centrifuged at 90,000 g for 18 h to study the delivery of TGF-α to the basolateralsurface [13] and the targeting of exosomes to the same surface domain [14] of MDCK cells. Themethod in Ref 14 provides a particularly impressive purification of vesicles containing Naked-2-enhanced green fluorescent protein). A 5-30% (w/v) iodixanol gradient centrifuged under the sameconditions was used in a study of miRNA effector proteins in exosomes derived from multivesicularbodies in monocytic cells [15].A simple flotation discontinuous density gradient can separate soluble cytosolic proteins from atotal vesicle fraction [21] For some recent publications of studies of exocytic vesicles in polarised cells see refs 22-24. 3. References 1. Leblanc, P., Alais, S., Porto-Carriero, I., Lehmann, S., Grassi, J., Raposo, G. and Darlix, J.L. (2006)Retrovirus infection strongly enhances scrapie infectivity release in cell culture EMBO J., 25, 2674-2685 2. Grigorov, B., Arcanger, F., Roingeard, P., Darlix, J-L. and Muriaux, D. (2006) Assembly of infectious HIV-1 in human epithelial and T-lymphoblastic cell lines J. Mol. Biol., 359, 848-862 3. Grigorov, B., Décimo, D., Smagulova, F., Péchoux, C., Mougel., M., Muriaux, D. and Darlix, J-L. (2007)Intracellular HIV-1 Gag localization is impaired by mutations in the nucleocapsid zinc fingersRetrovirology, 4:54 4. Jacobs, S.B.R., Basak, S., Murray, J.I. and Attardi, L.D. (2007) Siva is an apoptosis-selective p53 targetgene important for neuronal cell death Cell Death Differ., 14, 1374-1385 5. Spiczka, K.S. and Yeaman, C. (2008) Ral-regulated interaction between Sec5 and paxillin targets Exocystto focal complexes during cell migration J. Cell Sci., 121, 2880-2891 6. Chen, X-W., Inoue, M., Hsu, S. and Saltiel, A.R. (2006) RalA-exocyst-dependent recycling endosometrafficking is required for the completion of cytokinesis J. Biol. Chem., 281, 38609-38616 7. Lampugnani, M.G., Orsenigo, F., Gagliani, M.C., Tacchetti, C. and Dejana, E. (2006) Vascular endothelialcadherin controls VEGFR-2 internalization and signaling from intracellular compartments J. Cell Biol.,174, 593-604 8. Kolesnikova, L., Bamberg, S., Berghöfer, B. and Becker, S. (2004) The matrix protein of Marburg virus istransported to the plasma membrane along cellular membranes: exploiting the retrograde late endosomalpathway J. Virol., 78, 2382-2393 9. Hase, K., Kimura, S., Takatsu, H., Ohmae, M., Kawano, S., Kitamura, H., Ito, M., Watarai, H., Hazelett,C.C., Yeaman, C. and Ohno, H. (2009) M-Sec promotes membrane nanotube formation by interacting withRal and the exocyst complex Nat. Cell Biol., 11, 1427-1432 10. Yeh, T-Y.J., Meyer, T.N., Schwesinger, C., Tsun, Z-Y., Lee, R.M. and Chi, N-W. (2006) Tankyraserecruitment to the lateral plasma membrane in polarized epithelial cells: regulation by cell-cell contactand protein poly(ADP-ribosyl)ation Biochem J., 399, 415-425 11. Andersen, N.J. and Yeaman, C. (2010) Sec3-containing exocyst complex is required for desmosomeassembly in mammalian epithelial cells Mol. Biol. Cell, 21, 152-164 12. Wang, Q., Chen, X-W. and Margolis, B. (2007) PALS1 regulates E-cadherin trafficking in mammalianepithelial cells Mol. Biol. Cell, 18, 874-885 13. Li, C., Hao, M., Cao, Z., Ding, W., Graves-Deal, R., Hu, J., Piston, D.W. and Coffey, R.J. (2007) Naked2acts as a cargo recognition and targeting protein to ensure proper delivery and fusion of TGF-α-containing exocytic vesicles at the lower lateral membrane of polarized MDCK cells Mol. Biol. Cell 18,3081-3093 14. Cao, Z., Li, C., Higginbotham, J.N., Franklin, J.L., Tabb, D.L., Graves-Deal, R., Hill, S., Cheek, K.,Jerome, W.G., Lapierre, L.A., Goldenring, J.R., Ham, A-J.L. and Coffey, R.J. (2008) Use of fluorescence-activated vesicle sorting for isolation of Naked2-associated, basolaterally targeted exocytic vesicles forproteomics analysis Mol. Cell. Proteomics, 7, 1651-1667 15. Gibbings, D.J., Ciaudo, C., Erhardt, M. and Voinnet, O. (2009) Multivesicular bodies associate withcomponents of miRNA effector complexes and modulate miRNA activity Nat. Cell Biol., 11, 1143-1149 16. Yeaman, C., Grindstaff, K.K., Wright, J.R. and Nelson, W.J. (2001) Sec6/8 complexes on trans-Golginetwork and plasma membrane regulate stages of exocytosis in mammalian cells J. Cell Biol., 155, 593-604 17. Yeaman, C., Grindstaff, K.K. and Nelson, W.J. (2004) Mechanism of recruiting Sec6/8 (exocyst) complexto the apical juntional complex during polarization of epithelial cells J. Cell Sci., 117, 559-570
| Page 4 |
4
18.
Gromley, A., Yeaman, C., Rosa, J., Redick, S., Chen, C-T., Mirabelle, S., Guha, M., Sillibourne, J. andDoxsey, S.J. (2005) Centriolin anchoring of exocyst and SNARE complexes at the midbody is required forsecretory-vesicle-mediated abscission Cell, 123, 75-87
19.
Beronja, S., Laprise, P., Papoulas, O., Pellikka, M., Sisson, J. and Tepass, U. (2005) Essential function ofDrosophila Sec6 in apical exocytosis of epithelial photoreceptor cells J. Cell Biol., 169, 635-646
20.
Zhao, H., Ito, Y., Chappel, J., Andrews, N., Ross, F.P. and Teitelbaum, S.L. (2010) How do bone cellssecrete proteins? In Osteoimmunology, Adv. Exp. Med.Biol., 658 (ed. Choi, Y.), SpringerScience+Business Media, pp 105-109
21.
Jang, A., Lee, H-J., Suk, J-E., Jung, J-W., Kim, K-P. and Lee, S-J. (2010) Non-classical exocytosis of α-synuclein is sensitive to folding states and promoted under stress conditions J. Neurochem., 113, 1263–1274
22.
Caballero-Lima, D., Hautbergue, G.M., Wilson, S.A. and Sudbery, P.E. (2014) In Candida albicanshyphae, Sec2p is physically associated with SEC2 mRNA on secretory vesicles Mol. Microbiol., 94, 828–842
23.
Majumdar, R., Tavakoli Tameh, A. and Parent, C.A. (2016) Exosomes mediate LTB4 release duringneutrophil chemotaxis PLoS Biol., 14: e1002336
24.
Kreutzberger, A.J.B., Kiessling, V., Liang, B., Seelheim, P., Jakhanwal, S., Jahn, R., Castle, D. and Tamm,L.K. (2017) Reconstitution of calcium-mediated exocytosis of dense-core vesicles Sci. Adv., 3: e1603208
OptiPrepTM Application Sheet S47a 7th edition, January 2020