Axis Shield Lymphoprep
- Lymphoprep Tube can be used for the preparation of pure
lymphocyte suspensions for tissue typing, antilymphocyte
sera and immunological research. - Lymphoprep is manufactured, packed and released in
compliance with GMP and ISO13485.
Lymphoprep is a ready-made, sterile and endotoxin
tested solution with the following specifications:
- Sodium diatrizoate 9.1% (w/v)
Polysucrose 400 5.7% (w/v)
Density 1.077 ± 0.001 g/ml
Osmolality 290 ± 15mOsm
Endotoxins < 1.0
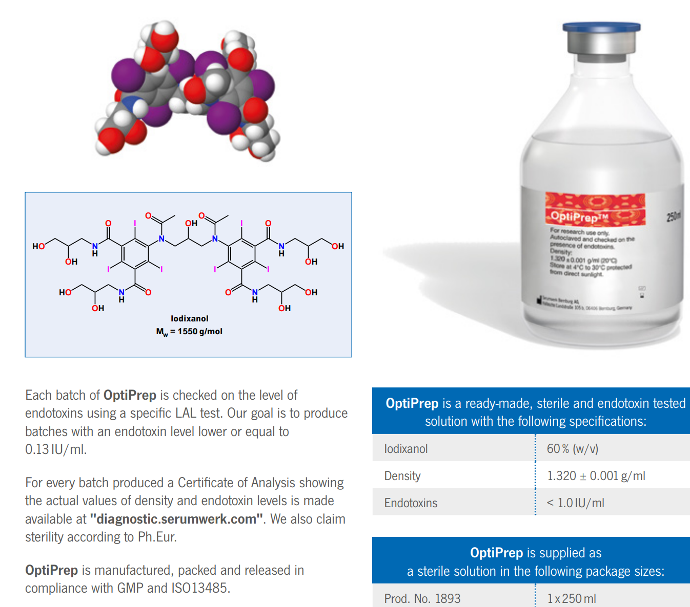
Iodixanol
Mw = 1550 g/mol
Lymphoprep protocol
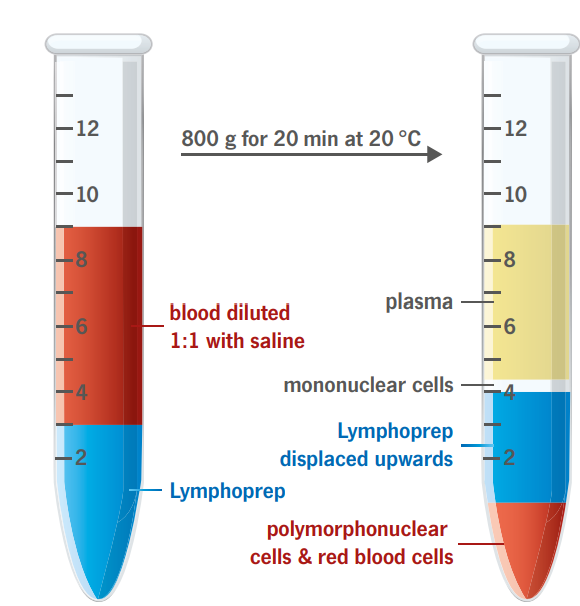
Mononuclear cells (monocytes and lymphocytes) have a lower buoyant density than the erythrocytes and the polymorphonuclear (PMN) leukocytes (granulocytes). The vast majority of mononuclear cells have densities below 1.077 g/ml. These cells can therefore be isolated by centrifugation on an isoosmotic medium with a density of 1.077 g/ml, which allows the erythrocytes and the PMNs to sediment through the medium while retaining the mononuclear cells at the sample/medium interface.
The described method is rapid, simple and reliable and gives excellent results with blood samples from normal individuals and patients.
To obtain the maximum yield it is important that the blood sample is diluted 1:1 with physiological saline before being applied to the gradient.
The contamination of erythrocytes in the mononuclear cell suspension is usually between 3-10 % of the total cell number.
Some immature PMNs may band with the lymphocytes during intense immunosuppressive therapy.
When heparinised blood is used, it is essential to remove most of the platelets, in order to avoid inhibition in the cytotoxicity test.
Optiprep
OptiPrep™
AXS-1114542
- OptiPrep is a sterile and endotoxin tested solution of 60% iodixanol in water with a density of 1.320 g/ml. Iodixanol was
developed as an X-ray contrast medium an has therefore been subjected to rigorous clinical testing. It is non-ionic, non-toxic
to cells and metabolically inert. Iodixanol solutions can be made isoosmotic at all useful densities, this solutions have low
viscosity and osmolality.
The high density of OptiPrep facilitates the fractionation of cells by flotation from a dense load zone through either
a continuous or discontinuous gradient or through a simple density barrier.
OptiPrep
The optimum density gradient medium
Applications
• Viruses
• Plasma lipoproteins
• Proteins and protein complexes
• Plasmid DNA
• Ribonucleoproteins
• Mammalian and non-mammalian cells
• Subcellular organelles
• Plasma membranes and domains
• Membrane vesicles and cytosol
• Organelles from non-mammalian sources
Isolation of:
• Membrane trafficking and cell signalling
Analysis of:
• Endocytosis and exocytosis
Axis-Shield Optiprep
Each batch of OptiPrep is checked on the level of
endotoxins using a specific LAL test. Our goal is to produce
batches with an endotoxin level lower or equal to
0.13 IU/ml.
OptiPrep is manufactured, packed and released in
compliance with GMP and ISO13485.
OptiPrep is supplied as a sterile solution in the following package sizes:
Prod. No. 1893 1 x 250ml
OptiPrep is a ready-made, sterile and endotoxin tested
solution with the following specifications:
- Iodixanol 60% (w/v)
Density 1.320 ± 0.001 g/ml
Endotoxins < 1.0 IU/ml
Lymphoprep Tube
Lymphoprep™ Tube
AXS-1019818/AXS-1019817
-
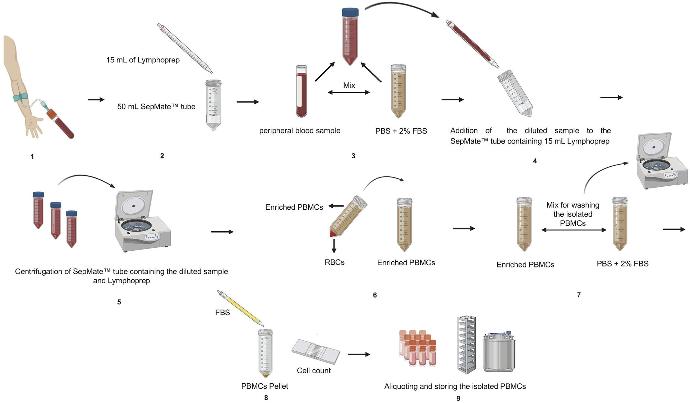
Separation of leucocytes from blood and bone marrow by
Lymphoprep Tube -
Isolation of human mononuclear cells – easy handling
-
Each batch of Lymphoprep is checked on the level of
endotoxins using a specific LAL test. -
Our goal is to produce
batches with an endotoxin level lower or equal to
0.13 IU/ml. -
For every batch produced a Certificate of Analysis showing
the actual values of density, osmolality and endotoxins are measured.
Axis-schield Lymphoprep Tube
- Sterile tube in which the LymphoPrep™ is contained below a plastic filter disc
- Highly effective for the purification of human mononuclear cells (monocytes and lymphocytes) from diluted blood
Please note: The Cat. No. of LymphoPrep™ Tube changes from 1019818 to 18001 but the product itself has not been changed in any way.
Therefore it is possible to receive the ordered products with Cat. No. 1019818 or 18001.
Ficoll of Lymphoprep Tube?
Although Lymphoprep and Ficoll are both com- posed of polysaccharides and diatrizoate, there are slight differences between the two density media . Ficoll-Paque contains edetate calcium disodium as a chelating agent, whereas Lymphoprep does not contain a chelating agent.
Polymorp hprep
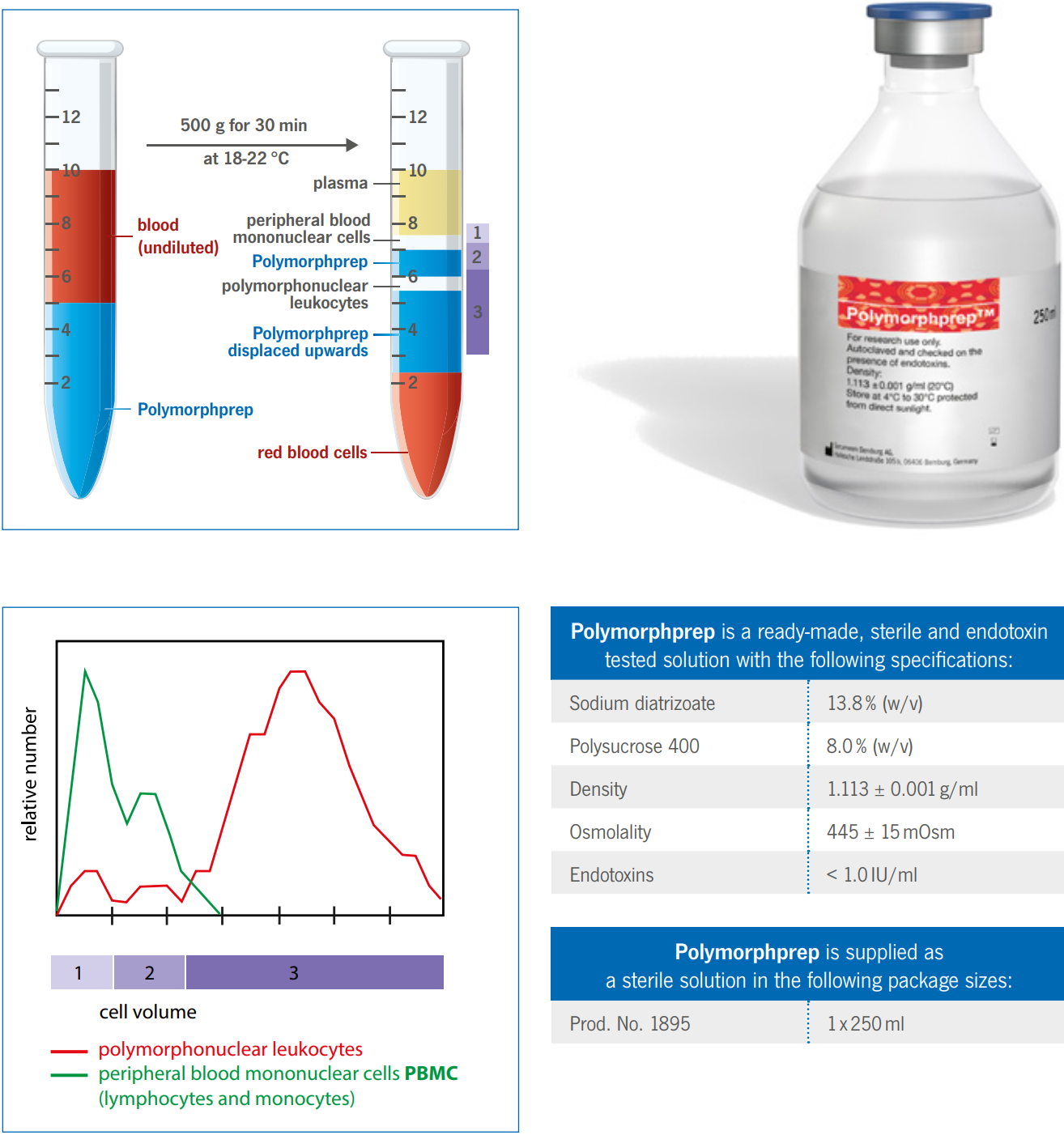
Behaviour of Erythrocytes during centrifugation with Polymorphprep
With the exception of the basophils, polymorphonuclear leukocytes (PMNs) have a much greater buoyant density than the
mononuclear cells, >1.085 g/ml. Unfortunately, the buoyant density of the erythrocytes tends to be from 1.09 – 1.11 g/ml,
this makes a separation from whole blood using a density barrier similar to that used for mononuclear cells more difficult.
A number of procedures have been developed in an effort to overcome these difficulties.
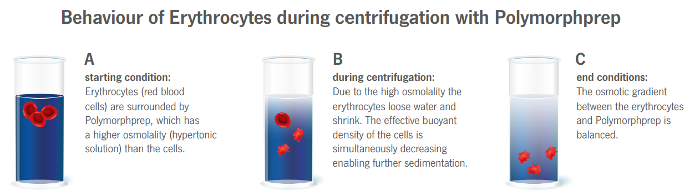
The high osmolality of Polymorphprep causes erythrocytes to lose water and shrink, thus increasing their effective
buoyant densities. This allows the aggregated erythrocytes to sediment rapidly through the dense medium.
Each batch of Polymorphprep is checked on the level of endotoxins using a specific LAL test. Our goal is to produce
batches with an endotoxin level lower or equal to 0.13 IU/ml.
The method is effective only with whole undiluted human blood not with a leukocyterich fraction or blood from
animal species.
The temperature is important to obtain optimal results, as changes in temperature effect the density and viscosity of the
Polymorphprep solution. The temperature of the blood sample and the medium should be kept between 18 - 22 °C.
Analysis of the top and bottom bands from the Polymorphprep separation using a Coulter STKR Cell Analyser is shown in
the figure on the right. The analyzer determines the number of cells in the sample (ordinate) as a function of cell volume
(abscissa). Relative cell number is the number of cells of a particular volume expressed as a fraction of the total in each
sample.
Polymorphprep
Isolation of human polymorphonuclear cells
Polymorphprep is supplied as
a sterile solution in the following package sizes:
Prod. No. 1895 1 x 250ml
Axis-Shield Polymorphprep
Polymorphprep is a ready-made, sterile and endotoxin tested solution with the following specifications:
Sodium diatrizoate 13.8% (w/v)
Polysucrose 400 8.0% (w/v)
Density 1.113 ± 0.001 g/ml
Osmolality 445 ± 15mOsm
Endotoxins < 1.0 IU/ml
Erythrocytes isolation
The cell band on top of the Polymorphprep contains only peripheral blood mononuclear cells (PBMCs). The cell band
itself can be separated into lymphocytes (upper layer, 1) and monocytes (lower layer, 2).
All of the PMNs are in the bottom cell band, which is enclosed of the Polymorphprep solution. Contamination of the PMN
band by erythrocytes is between 2 - 6% of the total cell number.
Nycodenz
Nycodenz is an off-white powder, freely soluble in water.
Nycodenz AXS-1002424
-
Solution up to 80% (w/v) with a density of 1.426 g/ml can be prepared.
-
It was originally developed as an X-ray contrast medium and have therefore been subjected to rigorous clinical testing.
• non-ionic, non-toxic to cells and metabolically inert
• can be used for the isolation of cells, subcellular organelles and membranes, macromolecules and viruses
• forms true solutions. It is therefore easy to remove the medium from the cells after fractionation
• is resistant to bacterial degradation
• solutions can be autoclaved
Gradients of Nycodenz can be generated by:
• centrifugation in situ (self-forming gradients).
• diffusion. Using Nycodenz, linear gradients can be simply prepared within 45 minutes.
• freezing and thawing.
• tilted tube rotation (Gradient Master™).
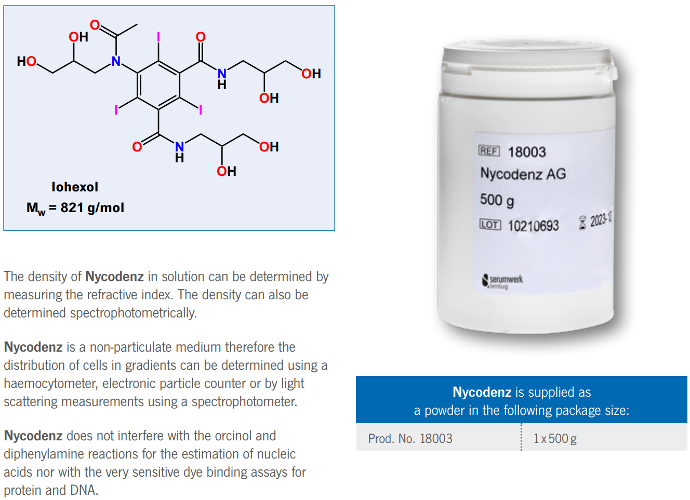
The density of Nycodenz in solution can be determined by
measuring the refractive index. The density can also be
determined spectrophotometrically.
Nycodenz is a non-particulate medium idem to Gentodenz therefore the
distribution of cells in gradients can be determined using a
haemocytometer, electronic particle counter or by light
scattering measurements using a spectrophotometer.
Nycodenz does not interfere with the orcinol and
diphenylamine reactions for the estimation of nucleic
acids nor with the very sensitive dye binding assays for
protein and DNA.
Polysaccharides and sugars can be determined in the presence of Nycodenz using the phenol/H2
SO4 assay. Fluorimetric assays of nucleic acids and proteins can also be carried out in the presence of Nycodenz. Nycodenz does not interfere
with most assays for the marker enzymes of subcellular components, also commercial scintillants are compatible with Nycodenz.
Nycodenz can be removed from samples by dialysis, ultrafiltration or gel filtration. Cells, subcellular organelles and
other particulate matter can be isolated from Nycodenz by centrifugation without the risk of contaminating the pellet
with Nycodenz.
Nycodenz
A universal density gradient medium
Nycodenz is supplied as
a powder in the following package size:
Prod. No. 18003 1 x 500 g
Applications
Isolation of:
• Mammalian and non-mammalian cells
• Subcellular organelles
• Organelles from non-mammalian sources
• Subcellular membranes
• Protein and protein complexes
• Ribonucleoproteins
• Viruses
Axis-Shield Nycodenz
Nycodenz is the trademark name for iohexol, whose systematic name is
5-(N-2,3-dihydroxypropylacetamido)-2,4,6-tri-iodo-N-N’-bis(2,3-dihydroxypropyl)isophthalamide.
It has a molecular weight of 821 g/mol. The chemical properties and stability of Nycodenz are related to its structure.
Its high density derives from the presence of a substituted triiodobenzene ring linked to a number of hydrophilic groups
which are responsible for the high water solubility of Nycodenz. It is a non-ionic derivative of metrizoic acid; the carboxyl
group present in metrizoic acid is linked to the amine group of 3-amino-1,2-propanediol. The dihydroxypropylacetamido side
chain is responsible for the very low toxicity of Nycodenz compared to metrizamide. Nycodenz has a defined melting point
between 174 and 180 °C. The iodinated aromatic nucleus absorbs strongly in the ultraviolet region of the spectrum with an
absorbtion maximum of 244 nm
Polysucrose
Polysucrose 400 is a synthetic high molecular weight polymer made by the copolymerization of sucrose and
epichlorohydrin. The molecules have a branched structure with a high content of hydroxyl groups giving a good solubility in
aqueous solutions.
The reactivity and stability of Polysucrose 400 are determined by its hydroxyl groups and the glycosidic bonds in the
sucrose residues.
Polysucrose 400 is stable in alkaline and neutral solutions. At pH values lower than 3, it is rapidly
hydrolysed, especially at elevated temperatures. In neutral solutions, Polysucrose 400 can be sterilized by autoclaving at
110 °C for 30 minutes without any degradation.
Polysucrose 400 is readily soluble in aqueous solutions when added slowly to the liquid with constant stirring.
Concentrations up to 50% (w/v) can easily be obtained.
- Polysucrose 400 A universal density gradient medium
- Prod. No. 7828 20 kg
Technical data
White odourless powder
Absorption <0.45
Specific optical rotation (α)
d 53° – 59°
Intrinsic viscosity (20 °C) 0.14 – 0.20
Average molecular weight (Mw) 450,000 ± 100,000 g/mol
Mw distribution by GPC conforms to standard
Loss on drying (%) <5.0%
pH (10% w/v aqueous solution) 7.0 – 9.0
Sulphated ash <0.3%
Content of chloride (ppm) <500 ppm
Sterilization test conforms
Microbiological contamination
<100CFU/g
<10 yeasts and mould/g
Bacterial endotoxins <10.0 IU/g
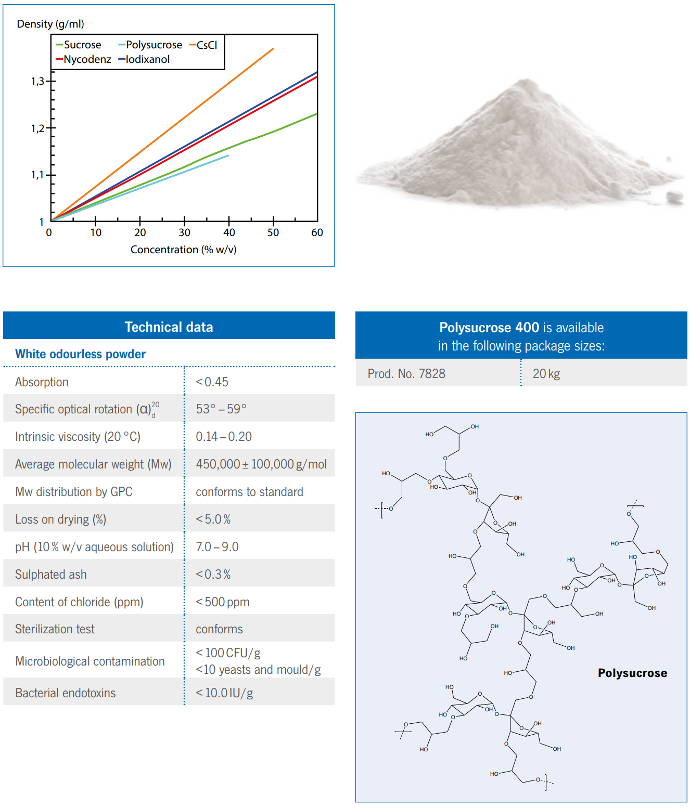
Axis-Shield Polysucrose
Using sodium metrizoate and a polysaccharide Bøyum (1968) developed a one-step centrifugal technique for
isolation of lymphocytes (Lymphoprep). In this method the polysaccharide aggregates the erythrocytes,
thereby increasing their sedimentation rate. Polysucrose 400 has also been used as a density gradient
medium for the purification of other cells and in membrane fractionation.
Non-ionic high molecular weight solutes such as polysucrose are required for a number of other research
scenarios. Polysucrose 400 may be used as a stabilizing agent in protein solutions and it can function as
an immunologically inert carrier for low molecular weight haptens in immunological studies. Polysucrose
400 is also used to reduce non-specific binding of labelled probes to nitrocellulose membranes during
nucleic acid hybridization. It also simplifies the loading of nucleic acids into the sample wells of agarose gels
for electrophoresis.

Albumin and Haemoglobin removal media


