4-(Imidazol-1-yl) phenol
N1-Me-pUTP, or N1-Methyl-Pseudo-UTP, is a modified nucleotide that can replace the natural cap of mRNA during in vitro transcription. This reduces the autoimmunity of mRNA and improves its expression efficiency by increasing ribosome binding. Compared to Pseudo-UTP, N1-Me-pUTP further improves mRNA expression efficiency and reduces autoimmunity.
Poly(A) Polymerase
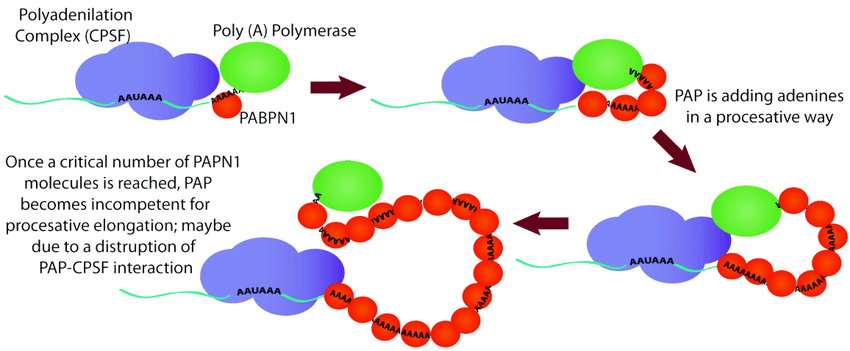
Poly(A) Polymerase, a recombinant E. coli polymerase, adds a polyadenylated tail to the 3' end of RNA, improving its stability and translation efficiency in eukaryotic cells. Poly(A) Polymerase has a wide range of applications in molecular biology, including in vitro transcription, mRNA expression, and RNA sequencing.
N1-Me-pUTP and Poly(A) Polymerase: Molecular Biology Reagents for Improved mRNA Expression
N1-Me-pUTP is a modified nucleotide that can replace the natural cap and is added to in vitro transcription to reduce the autoimmunity of mRNA and improve its expression efficiency. It does this by increasing the abundance of ribosome binding. Compared to Pseudo-UTP, N1-Me-pUTP can further improve the expression efficiency of mRNA in cells and reduce its autoimmunity.
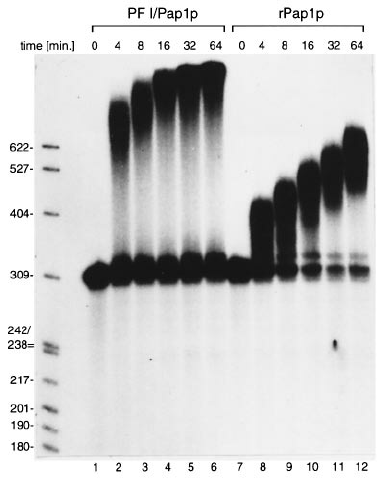
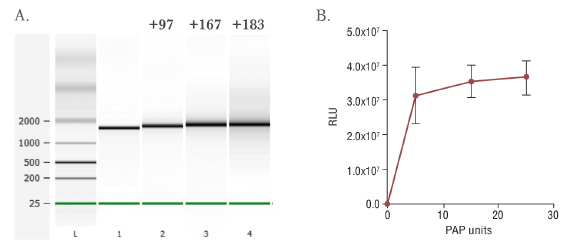
Poly(A) Polymerase is an enzyme that adds a polyadenylated tail to the 3' end of RNA. This structure can improve the stability of RNA and its translation efficiency in eukaryotic cells. Poly(A) Polymerase has high tailing efficiency and can add 20-200 A bases to the 3' end of RNA.
Together, N1-Me-pUTP and Poly(A) Polymerase are powerful tools for improving the expression of mRNA in vitro and in cells. They can be used to produce mRNA that is more stable, has less autoimmunity, and is translated more efficiently. This makes them valuable tools for a variety of applications, such as gene therapy, protein expression, and vaccine development.
Additional Information:
- N1-Me-pUTP has a purity of ≥99% (HPLC), a concentration of 100 mM, and a pH (25°C) of 6.9 to 7.1. It has been tested for DNase, RNase, and nuclease contamination, and it is free of all three.
- Poly(A) Polymerase is a polymerase expressed by recombinant E.coli. It does not depend on a template and can catalyze the sequential incorporation of ATP into the 3' end of RNA in the form of AMP.
N1-Me-Pseudo UTP sodiumsolution
| Product Name | Cat. No. | Size |
|---|---|---|
| N1-Me-Pseudo UTP sodiumsolution | HBP002500 | 0.1mL |
| HBP002501 | 1mL | |
| HBP002502 | 20mL | |
| HBP002503 | 100mL | |
| HBP002504 | 500mL |
Poly(A) Polymerase
| Product Name | Catalog | Size |
|---|---|---|
| Poly(A) Polymerase | HBP000801 | 0.1 KU |
| HBP000802 | 0.5 KU | |
| HBP000803 | 5 KU | |
| HBP000804 | 50 KU |
Specification
Shipping and storage
Shipping on ice packs. Store at -25~-15℃, avoid repeated freezing and thawing, valid for 2 years.
Precautions
1) After the product is dissolved, it should be stored on ice, and it should be stored at -20°C immediately after use.
2) For your safety and health, please wear a lab coat and disposable gloves to operate.
Specification
Product Component
| Component | HBP000801 | HBP000802 |
|---|---|---|
| E.coli Poly(A) Polymerase (5 U/μL) | 0.02mL | 0.1mL |
| 10 × Poly(A) Polymerase Buffer | 1.5mL | 1.5mL |
| ATP(10 mM) | 0.2mL | 0.2mL |
| Component | HBP000803 | HBP000804 |
|---|---|---|
| E.coli Poly(A) Polymerase (5 U/μL) | 1mL | 10mL |
| 10 × Poly(A) Polymerase Buffer | 15mL | 150mL |
| ATP(10 mM) | 2mL | 20mL |
Storage and transportation
Store at -20 ℃, Transportation by dry ice.
Unit Definition
One unit is defined as the amount of enzyme that will incorporate 1 nmol of AMP into RNA in a 20 µl volume in 10 minutes at 37°C.
Quality Control
Purity≥95%, no DNase, RNase activity, host DNA residue≤100pg/mg, host protein residue≤50ppm, endotoxin residue≤10EU/mg, no protease activity, sterile, no mycoplasma.
Reaction System and Condition
| Component Name | Amount |
|---|---|
| 10 × Poly(A) Polymerase Buffer | 2 μL |
| E.coli Poly(A) Polymerase (5 U/μl) | 0.2 - 2 μl |
| ATP (10 mM) | 1 μl |
| Denatured Cap0 RNA | 10 μg |
| RNase-free ddH2O | Up to 20 μL |
Notes
- The length of the Poly(A) tail added in this reaction is affected by factors such as enzyme amount, ATP concentration and reaction time. Generally, this enzyme can add ~30 A bases for 30min at 37℃, and ~100 A bases in 60min. .
- The enzyme adds AMP to the 3′ end of RNA with high selectivity, and does not add the same length of Poly(A) to all RNA molecules.
- The substrate of this enzyme can only be RNA, and it needs divalent ions such as Mg2+ to be active.
- This enzyme can also be reacted with M-MuLV Reverse Transcriptase Reaction Buffer
Elevating mRNA Expression: Unveiling the Potential of N1-Me-pUTP and Poly(A) Polymerase
Introduction:
In the dynamic realm of molecular biology, the quest for precise and enhanced mRNA expression is met with the innovative synergy of N1-Me-pUTP and Poly(A) Polymerase. These reagents represent a transformative duo, each playing a distinct yet complementary role in optimizing the mRNA synthesis process. Let's delve into the extraordinary capabilities of these reagents and how they collectively elevate mRNA expression.
N1-Me-pUTP: A Catalyst for Precision:
1. Precision in mRNA Labeling: N1-Me-pUTP, a modified nucleotide analog, stands at the forefront of mRNA expression enhancement. Its unique chemical structure allows for precise labeling during in vitro transcription, providing researchers with a powerful tool to tag and trace mRNA molecules with unparalleled accuracy.
2. Stability and Resistance: Beyond its labeling prowess, N1-Me-pUTP enhances mRNA stability. The modified nucleotide imparts increased resistance to degradation, ensuring the longevity of synthesized mRNA transcripts. This stability is paramount for downstream applications such as RNA-protein interaction studies and functional assays.
Poly(A) Polymerase: Tailoring mRNA for Superior Expression:
1. Tail Addition for Enhanced Stability: Poly(A) Polymerase steps in to complement the process by adding a poly(A) tail to the 3' end of the synthesized mRNA. This tail addition not only enhances mRNA stability by protecting it from exonuclease degradation but also facilitates efficient translation and nuclear export.
2. Increased mRNA Half-Life: Poly(A) Polymerase extends the mRNA half-life, a critical factor in maintaining a robust pool of functional transcripts. This prolonged stability ensures that the mRNA remains available for translation over an extended duration, optimizing protein production.
Advantages of the Synergistic Approach:
1. Synergistic Enhancement: The combination of N1-Me-pUTP and Poly(A) Polymerase creates a synergistic effect, maximizing the efficiency of mRNA expression. The precision of N1-Me-pUTP labeling, coupled with the stability conferred by Poly(A) tail addition, results in a potent tandem that significantly amplifies the overall mRNA expression yield.
2. Versatility in Applications: This synergistic approach is versatile and adaptable to a spectrum of applications. From mRNA labeling for fluorescence in situ hybridization (FISH) to the generation of high-quality mRNA for in vitro translation, the reagents cater to diverse experimental needs with unwavering efficacy.
Applications in Genomic Research:
1. Functional Genomics: N1-Me-pUTP and Poly(A) Polymerase find their applications in functional genomics, empowering researchers to precisely label and tailor mRNA for studies that unravel the functional aspects of genes and their regulatory elements.
2. Transcriptomics: In the realm of transcriptomics, this dynamic duo contributes to the generation of enriched mRNA libraries, facilitating a deeper understanding of gene expression patterns, alternative splicing events, and RNA processing dynamics.
Conclusion:
N1-Me-pUTP and Poly(A) Polymerase stand as avant-garde reagents, redefining the landscape of mRNA expression enhancement. Their individual capabilities, when harnessed in tandem, open avenues for unprecedented precision, stability, and versatility in mRNA synthesis. As the synergy of these reagents unfolds, researchers find themselves equipped with a powerful toolkit for advancing genomic research, functional genomics, and transcriptomics, unlocking the intricacies of gene expression with heightened precision and efficiency.